
Accelerate IDMP Compliance, Simplify Reference Data Management
Readily stay up-to-date with ever-changing ISO IDMP standards and health authority reference data requirements.
SPORIFY
SPORIFY enables greater harmonization of data across systems and geographies by accelerating and simplifying how you match, maintain, synchronize, and integrate reference data.
Centralized Location for IDMP Compliance
Prepare and maintain compliant product data through real-time SPOR updates to keep pace with emerging ISO IDMP standards.
Seamless RIM Integration
SPORIFY is a vendor-agnostic application that connects via lightweight APIs and is compatible with any RIM system, eliminating the need for migration.
Increased Productivity and Time Savings
Complete initial data mapping up to 9X faster and use automated data synchronization to stay aligned with changes.
Simplified Reference Data Management
Significantly reduce manual effort needed to locate source data and translate internal controlled vocabularies to SPOR RMS and OMS.
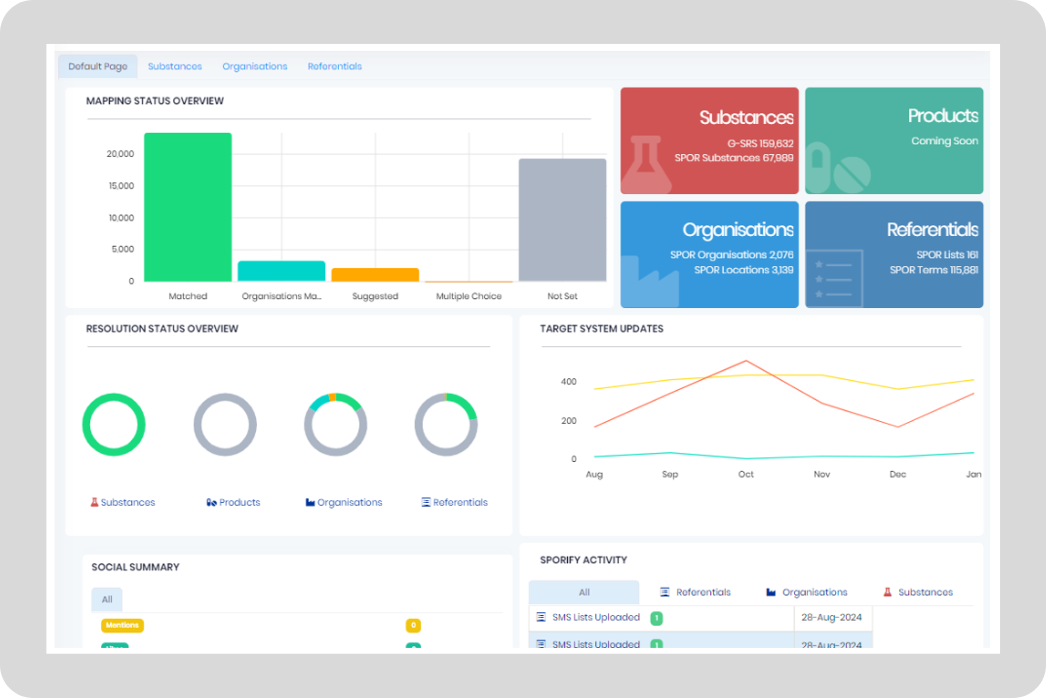
Features
Deliver real value with SPORIFY.

EMA SPOR Mapping and Integration
APIs provide a simple and secure solution to enable mapping, sync and notification services between your systems and SPOR master data.

User-Friendly Data Insights
Gain real-time visibility into compliance status across your operations, in addition to dashboards and analytics to optimize operational performance.

Automatic Data Sync and Notifications
Maintain compliant data even as health authority data changes with automated workflow triggers and notifications that inform you of needed changes.
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 500 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved
