Your Solution for Quality Process Management
Ensure harmonized quality management with end-to-end, purpose-built processes.
LifeSphere® Technology is trusted by these industry leaders:







Quality Management System (QMS)
Quality Management System uses a pre-configured set of business processes incorporating best practices across the R&D drug development lifecycle to solve challenges in R&D, Clinical, Regulatory, Pharmacovigilance, and Manufacturing.
Global Harmonization
Harmonize your end-to-end quality management processes in a single system, including through all departments and subsidiaries.
Minimize Risk
Monitor and control quality processes to ensure more efficient and consistent global compliance and minimize potential non-compliance.
Digital Control
Implement standardized and controlled processes to increase inspection and audit readiness and eliminate uncontrolled workflows.
Process Insights
Make proactive and informed decisions around quality management with improved quality reporting and greater transparency into quality processes.
Features
Deliver real value with Quality Management System.

Quality Event Management
Manage deviations, complaints, non-conformances, out-of-specification results, and more.

End-to-End Processes
Support for processes including CAPA, investigation and root cause analysis, change control, supplier qualification, and more.

Audit Capabilities
Create, track, and approve audit plans, activities, agendas, reports, and more.

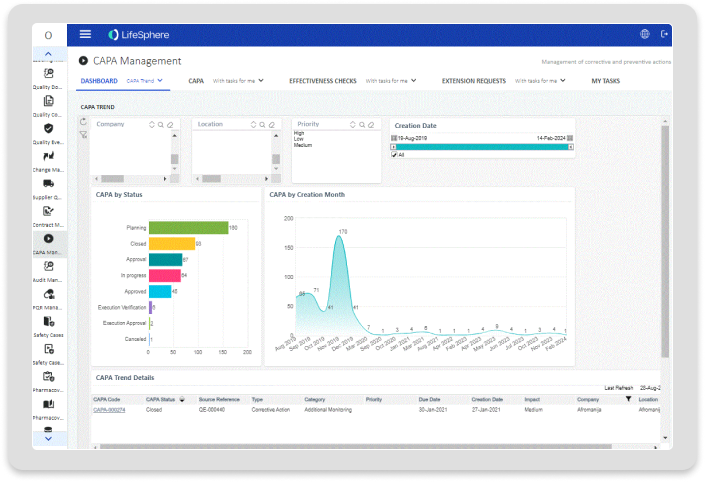
Process Dashboards
Dashboards providing actionable quality process management insights.

Global Scalability
Our solution is highly suitable for global rollouts, with a proven track record of global implementations, including support for more than 18 languages.

A Single Vendor
Establish a single, end-to-end vendor by integrating with your larger ecosystem, including safety, medical information, and CRM systems.
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 500 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved
