Accelerated Health Authority Interactions
Automate intake and management of HA communications, powered by LifeSphere NavaX™.
LifeSphere® Technology is trusted by these industry leaders:







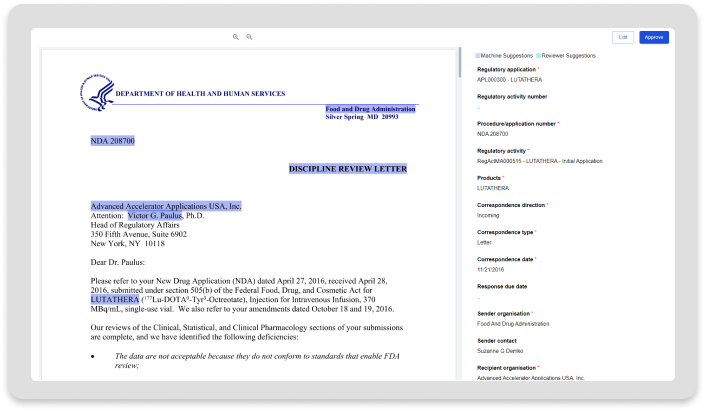
Health Authority Interactions
Health Authority Interactions accelerates the intake of Health Authority (HA) communications and builds a knowledgebase driving transparency and consistency with HA interactions.
Accelerated Intake
Leverage LifeSphere NavaX advanced automation capabilities to ingest and process HA communications.
Increased Consistency
Reduce error-prone and disjointed manual processes for managing HA interactions.
Greater Transparency
Build a central knowledgebase that enables you to readily understand the latest HA interaction status.
Advanced Insights
Leverage LifeSphere NavaX advanced automation for robust and up-to-date insights into HA interactions.
Features
Deliver real value with Health Authority Interactions.

HA Intake
Automate the ingestion of HA communications.

HA Processing
Automate the classification and extraction of relevant metadata.

Managed Information
Access HA information in a single, centralized repository.

Response Drafting
Automate the drafting of HA responses.

Knowledgebase Querying
Perform flexible searching and filtering of repository resources.

Advanced Cognition
Provides advanced conversational information discovery via chatbot.
Related Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 500 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved
