Labeling Management
Drive more efficient and consistent labeling management processes across your operations.
LifeSphere® Technology is trusted by these industry leaders:







Labeling
Labeling enables you to streamline your labeling workflows through end-to-end labeling change management and compliance tracking.
Work More Efficiently
Increase productivity and reduce rework with streamlined and automated labeling management workflows.
Enhanced Compliance
Minimize regulatory non-compliance risk with enhanced control over labeling changes and their implementation.
End-to-End Management
Make it simple to manage, reuse, and control labeling data and documents throughout their lifecycle.
Greater Visibility
Gain added insights into labeling status and changes with enhanced compliance tracking.
Features
Deliver real value with Labeling.

Process Management
Support local and global end-to-end labeling processes.

Change Management
Support the review, tracking, and implementation of labelling changes.

Workflow Automation
Enable automated notifications and process triggers.

Cross-Functionality
Establish workflows that span processes and departments.

Downstream Integration
Seamless connection to the submission and approval processes connected to the end-to-end RIM workflow.

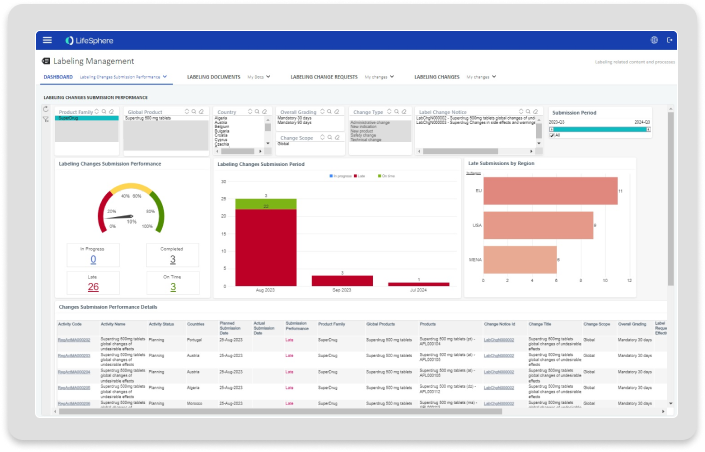
Dashboarding
Interactive dashboards for visibility into labeling changes and actions needed.
Related Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 500 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved
