Smarter, Faster Literature Management
Transform medical literature screening with cutting-edge GenAI automation.
LifeSphere® Technology is trusted by these industry leaders:







Literature Intelligence
Literature Intelligence is our system-agnostic, GenAI-powered solution for literature intake and assessment, purpose-built to automate workflows for increased efficiency and data accuracy.
Unmatched Efficiency
Cut literature screening from days to minutes by replacing repetitive, labor-intensive manual processes with powerful, end-to-end GenAI-powered automation.
Top-Quality Compliance
Ensure consistent compliance by eliminating human-error, fatigue, and bias when working to meet regulatory requirements.
Smarter Literature Management
Harness technology to retrieve literature data swiftly and effortlessly, lighten workloads, and shift focus to higher-value activities.
Overhead Reduction
Lower total cost of ownership through a scalable cloud solution that offers system-agnostic integration with drug safety systems.
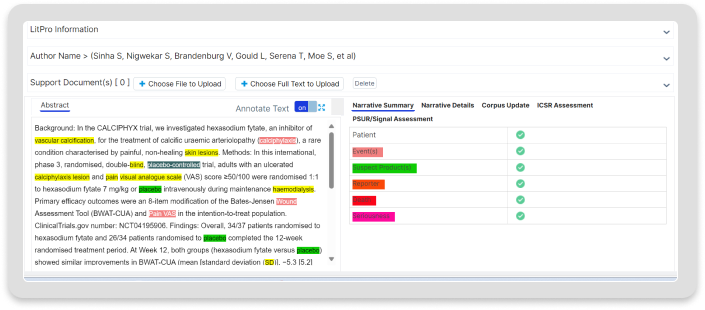
Features
Deliver real value with Literature Intelligence.

Advanced Automation
Leverage cutting-edge, production-ready AI/ML and GenAI automation capabilities that lead to major efficiencies, reduced errors, and improved decision-making.

System-Agnostic Integration
Ensure seamless interoperability with existing safety systems to optimize workflows and enable more seamless data flow without disruption.

API integration with Global Databases
Enable easy, automated literature ingestion and de-duplication from PubMed, Medline, and Embase, for comprehensive and accurate data flow.

Enhanced Literature Processing
Automate de-duplication and ICSR relevancy assessment to process large volumes of literature more efficiently and minimize manual intervention.

Integration with Preferred Full-Text Providers
Enhance data extraction and in-depth analysis of the full-text articles with GenAI for faster and more accurate insights.

Tailored Workflows
Gain the flexibility to meet specific organizational needs while ensuring full compliance with global regulatory standards.
Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 500 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved
